Queensland researchers have shown that single crystals, typically thought of as brittle and inelastic, are flexible enough to be bent repeatedly and even tied in a knot.
Researchers from Queensland University of Technology and The University of Queensland (UQ) determined and measured the structural mechanism behind the elasticity of the crystals down to the atomic level using the Australian Synchrotron.
Video: Copyright QUT
Their work, published in Nature Chemistry, opens the door for the use of flexible crystals in applications in industry and technology.
The research was led by ARC Future Fellows Associate Professor Jack Clegg in UQ’s School of Chemistry and Molecular Biosciences and Associate Professor John McMurtrie in QUT’s Science and Engineering Faculty.
Associate Professor McMurtrie said the results challenged conventional thinking about crystalline structures.
“Crystals are something we work with a lot – they’re typically grown in small blocks, are hard and brittle, and when struck or bent they crack or shatter,” he said.
“While it has previously been observed that some crystals could bend, this is the first study to examine the process in detail.
“We found that the crystals exhibit traditional characteristics of not only hard matter, but soft matter like nylon.”
The researchers grew bendable crystals about the width of a fishing line and up to five centimetres long from a common metal compound – copper (II) acetylacetonate.
They mapped changes in the atomic scale structure when the crystals were bent using X-ray measurements performed at the Australian Synchrotron.
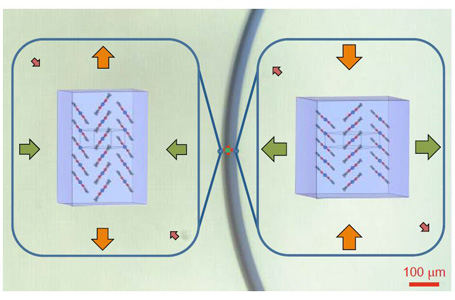
Dr Jason Price, a beamline scientist at the Australian Synchrotron, who helped design the experiments, explained that preliminary studies were undertaken on the MX1 beamline and followed by extensive measurements on the MX2 beamline.
“This experiment really shows the opportunity to look at variation within a single crystal. The microfocus capacity of the beam at MX2 is such that it can gather data from different parts of the crystal, so, when it is under strain from expansion or compression.”
Crystals from six other structurally related compounds, some containing copper and some other metals, were also tested and found to be flexible.
Associate Professor Clegg said the experiments showed that the crystals can be repeatedly bent and return quickly to their original shape with no signs of breaking or cracking when the force bending them is removed.
“Under strain the crystal molecules reversibly rotate and reorganise to allow the compression and expansion required for elasticity and still maintain the integrity of the crystal structure,” he said.
“The ability of crystals to bend flexibly had wide-ranging implications in industry and technology.
“Crystallinity is a property that underpins a variety of existing technologies, including semi-conductors and lasers which are used in almost every electronic device from DVD players to mobile phones and computers.
“But the hardness that makes them suitable for high-strength industrial components limits their use in other technologies. Flexible crystals like these could lead to new hybrid materials for numerous applications from components of planes and spacecraft to parts of motion or pressure sensors and electronic devices.”
Associate Professor McMurtrie said the method the researchers have developed to measure the changes during bending could also be used to explore flexibility in any other crystals.
“This is an exciting prospect given that there are millions of different types of crystals already known and many more yet to be discovered,” he said.
“Bending the crystal changes its optical and magnetic properties, and our next step is to explore these optical and magnetic responses with a view to identifying applications in new technologies.”
The research was funded by an ARC Discovery Grant and supported by the Australian Synchrotron. Research collaborators and co-authors of the study are: Anna Worthy, Professor Chen Yan, and Yanan Xu (QUT) and Dr Arnaud Grosjean, Dr Michael Pfrunder and Dr Grant Edwards (UQ).
http://dx.doi.org/10.1038/nchem.2848
For interview:
QUT Associate Professor John McMurtrie, j.mcmurtrie@qut.edu.au 07 3138 1220
UQ Associate Professor Jack Clegg, j.clegg@uq.edu.au 0408 642082
