A giant, hollow, protein shell manufactured by some bacteria and used to contain toxins that kill insects could lead to new bio-insecticides for controlling crop pests.
 Discovered recently by a team of Australian and New Zealand researchers, the protein canister protects the Yersinia entomophaga bacterium from its own toxins and keeps them safe until specific environmental conditions are encountered. The insect-killing bacterium is related to the infamous Yersinia bacterium that is thought to have caused the Black Death plagues in Europe in the 14th to 17th centuries. The gene sequence that codes for the hollow, protective structure is found in other bacteria and animals too, but the contents of the canister can vary quite widely. The researchers believe they have found a ‘molecular assembly manual’ for the protection of toxic or sensitive molecules – with implications for research into human disease as well as pesticides.
Discovered recently by a team of Australian and New Zealand researchers, the protein canister protects the Yersinia entomophaga bacterium from its own toxins and keeps them safe until specific environmental conditions are encountered. The insect-killing bacterium is related to the infamous Yersinia bacterium that is thought to have caused the Black Death plagues in Europe in the 14th to 17th centuries. The gene sequence that codes for the hollow, protective structure is found in other bacteria and animals too, but the contents of the canister can vary quite widely. The researchers believe they have found a ‘molecular assembly manual’ for the protection of toxic or sensitive molecules – with implications for research into human disease as well as pesticides.
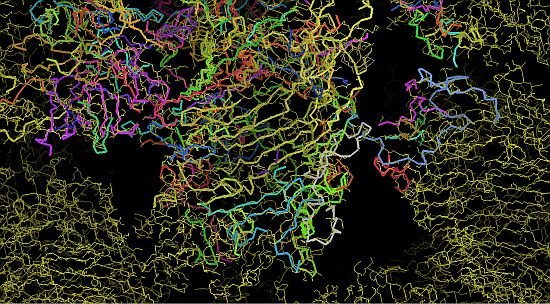 Dr Santosh Panjikar from the Australian Synchrotron’s macromolecular crystallography beamline is one of the co-authors of a paper reporting the latest research findings in the prestigious science journal Nature in August 2013. Santosh took x-ray data collected by Jason Busby and Shaun Lott (AgResearch Structural Biology Laboratory, University of Auckland) from crystals of the large complexes at the micro-focus beamline (MX2) at the Australian Synchrotron, and used Auto-Rickshaw and the MASSIVE high-performance computing cluster to solve the challenging structure.
Dr Santosh Panjikar from the Australian Synchrotron’s macromolecular crystallography beamline is one of the co-authors of a paper reporting the latest research findings in the prestigious science journal Nature in August 2013. Santosh took x-ray data collected by Jason Busby and Shaun Lott (AgResearch Structural Biology Laboratory, University of Auckland) from crystals of the large complexes at the micro-focus beamline (MX2) at the Australian Synchrotron, and used Auto-Rickshaw and the MASSIVE high-performance computing cluster to solve the challenging structure.
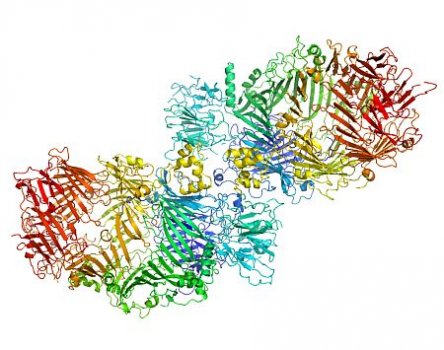 “My role was to suggest a strategy for x-ray data collection from derivative crystals of the large complex protein, and in processing and scaling of the data set,” Santosh said. “Determination of the crystal structure was extremely challenging, but I found an unusual strategy to retrieve and improve the phase information for interpretable electron density map.”
“My role was to suggest a strategy for x-ray data collection from derivative crystals of the large complex protein, and in processing and scaling of the data set,” Santosh said. “Determination of the crystal structure was extremely challenging, but I found an unusual strategy to retrieve and improve the phase information for interpretable electron density map.”
Jason N. Busby, Santosh Panjikar, Michael J. Landsberg, Mark R. H. Hurst & J. Shaun Lott, The BC component of ABC toxins is an RHS-repeat-containing protein encapsulation device, Nature (04 August 2013) | doi:10.1038/nature12465
University of Queensland media release
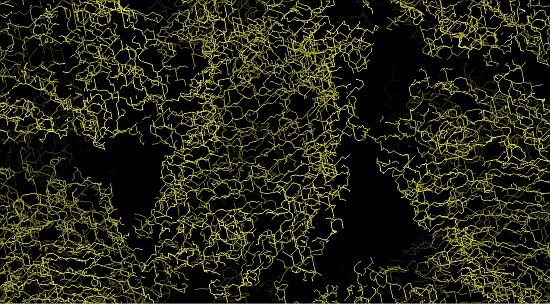
Images at right, top to bottom: (i) partway through phasing, (ii) the protein structure begins to take shape, (iii) the final protein structure. Images: Santosh Panjikar
