Australian researchers have made a significant discovery about the biological mechanisms controlling the electrical currents that underpin sensory perception and nervous system function in humans.
A collaborative research team from the Walter and Eliza Hall Institute (WEHI) and the Victor Chang Cardiac Research Institute has made a significant discovery about the mechanisms controlling electrical currents in the human body, using the facilities at the Australian Synchrotron.
Electrical currents within the human body involve coordinated interplay between different types of ion channels and underpin most bodily functions. Potassium ions and other ions carry charge, and as they pass through channels in the membrane the flow of particles can be measured as an electrical current.
“Potassium currents across cell membranes are important in many cellular processes, particularly those involving communication between cells,” says Jacqui Gulbis from WEHI. “They play an essential role in the electrical signalling underlying organ function, sensory perception and neurotransmission. In the heart, for example, contractions occur in response to the rhythmic ebb-and-flow of potassium. Specialised pores responsible for these currents, known as potassium channels, are highly selective for potassium over other ions.
While previous studies have revealed the appearance and electrical properties of potassium channels, there is still only a very limited understanding of the molecular mechanisms by which conduction is switched off and on in response to regulatory signals.
Now Jacqui and her colleagues have used the Australian Synchrotron to obtain a new perspective on the mechanisms that allow these channels to control the flow of potassium currents across cell membranes – providing new and exciting insights into the control of some crucial functions such as sensory perception.
All potassium channels have a common pore structure with a characteristic region known as the ion selectivity filter. This region of the channel enables potassium, to permeate the membrane, while blocking the passage of sodium, and is responsible for conducting signals from cell to cell, in particular for the nervous system.
Jacqui and her colleagues used the MX2 micro-crystallography beamline at the Australian Synchrotron to identify a ‘molecular gate’ in the ion selectivity filter – revealing that changes in remote regions of the channel assembly enable the selectivity filter to act as an on/off switch in response to physiological signals, alternately curbing or permitting the flow of potassium ions. Their findings indicate that the selectivity filter may play a significant role in the gating process. Different families of channels are distinguishable on the basis of the assemblies that regulate their activity, and the study also reveals the mechanism by which members of the Kir family of potassium channels control the direction of potassium flow. Kir channels have many essential roles, such as in cardiac activity and the secretion of insulin from pancreatic beta cells, and were employed in the study as a model system for studying general principles of potassium channel gating.
The group’s findings were made possible by continued regular access to synchrotron sources, and most of their data was collected at the Australian Synchrotron. Crystals of integral membrane proteins such as potassium channels typically have a higher aqueous content and ‘softer’ contacts between the individual molecules than do crystals of naturally soluble proteins, leading to weak, poor quality diffraction data that cannot easily be collected on laboratory systems. The microfocus optics on the MX2 beamline provided the x-ray flux and intensity needed to collect diffraction data from the crystals, while the narrow focus enabled sampling of different regions of each crystal, helping the team to overcome these technical challenges and progress their important research.
The next steps in the project will also rely on synchrotron access: testing some of the hypotheses derived from the study, using a combination of structural and functional data from potassium channels with small changes engineered into the structure.
The work was published in Cell on 3 June 2010. 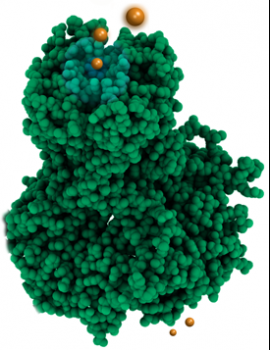
Potassium ions (orange) shown entering a Kir potassium channel. In living systems the upper lobe representing the pore would be embedded in a cell membrane, while the lower lobe is suspended within the cell. (Figure: Jacqui Gulbis)
