Recent work by Australian Synchrotron and university researchers could lead to improved methods for separating ‘chiral’ drug mixtures, with potential longer-term benefits including more-effective pharmaceutical drugs for consumers.
Most biological molecules occur in both left- and right-handed non-identical mirror images known as chiral enantiomers. Drug synthesis often produces chiral pairs: equal quantities of both left- and right-handed forms, but typically only one of these forms will be effective. In some cases the other form may even be harmful, as was the case with thalidomide, where one form was a sedative but the other form caused birth defects.
Anton Tadich and Lars Thomsen from the Australian Synchrotron and their collaborators at La Trobe University and the University of Newcastle have discovered a way to determine the chiral orientation of metal substrates that show significant potential for separating chiral drug pairs. Their work was featured recently in Physics Today online and will appear shortly in Physical Review Letters, the peer-reviewed journal of the American Physical Society.
Anton and his collaborators studied a chiral copper substrate using photoemission techniques on the soft x-ray beamline at the Australian Synchrotron. They also used a special angle-resolving photoemission spectrometer built by La Trobe University Physics Department and located at the BESSY synchrotron in Berlin, Germany. They then compared their photoemission distribution results with geometric lattice projections of the substrate structure to determine which of the two possible chiral structures was correct.
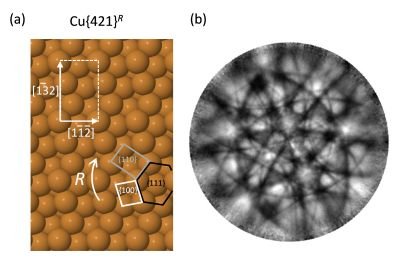
(b): emission distribution of photoelectrons excited from a chiral copper surface, Cu{421} (shown in (a)). The pattern allows researchers to identify the precise chiral orientation of the copper surface, which is important for understanding how the surface reacts with chiral molecules.
