Sample Stages
The configuration of the beamline endstation is fixed such that all user groups employ a standardised gonio head for mounting their crystals.
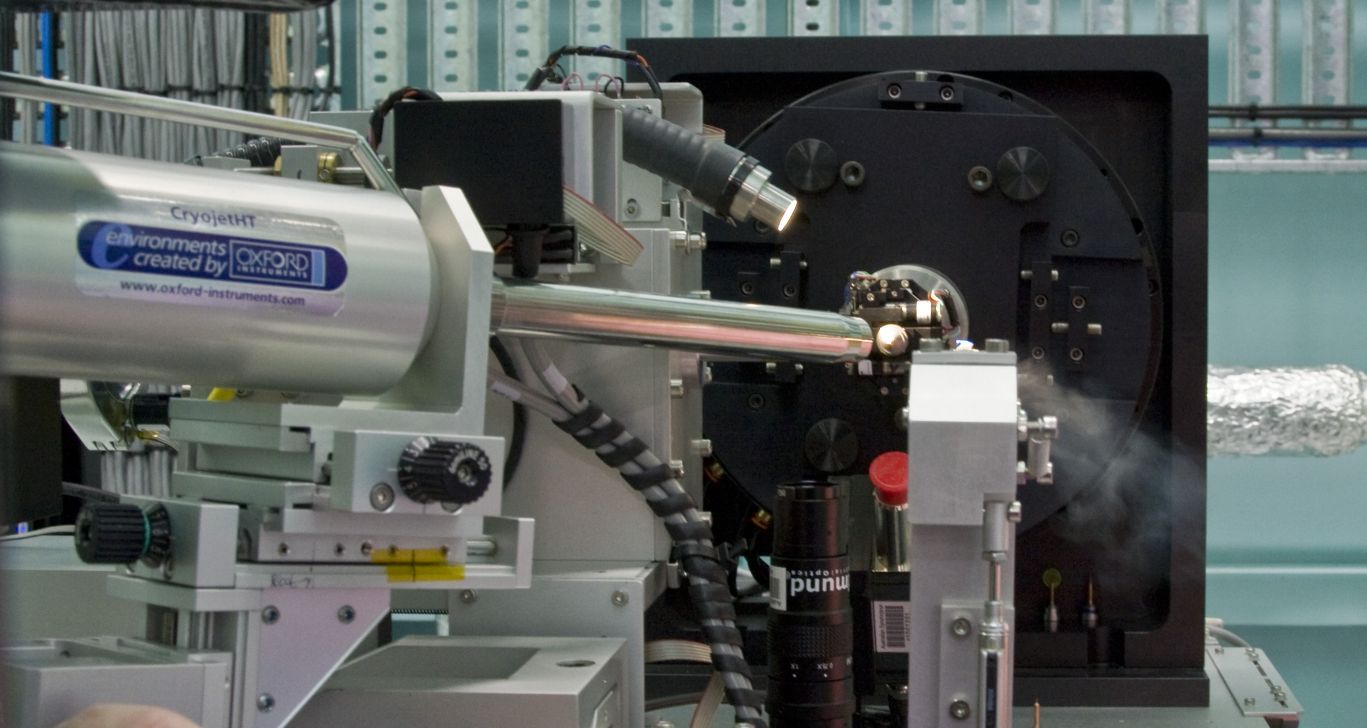 |
|
Pay special attention to the following:
-
If running remote access or using the robot, only use sample pins that are compatible with the automated loading robot (SAM) as detailed above. SPINE PINS are not appropriate for the MX1 or MX2 SAM automated robots and have the potential to seriously damage the robot toolset and grippers! Users MUST avoid loading SPINE pins in any cassettes that are brought to the AS beamlines.
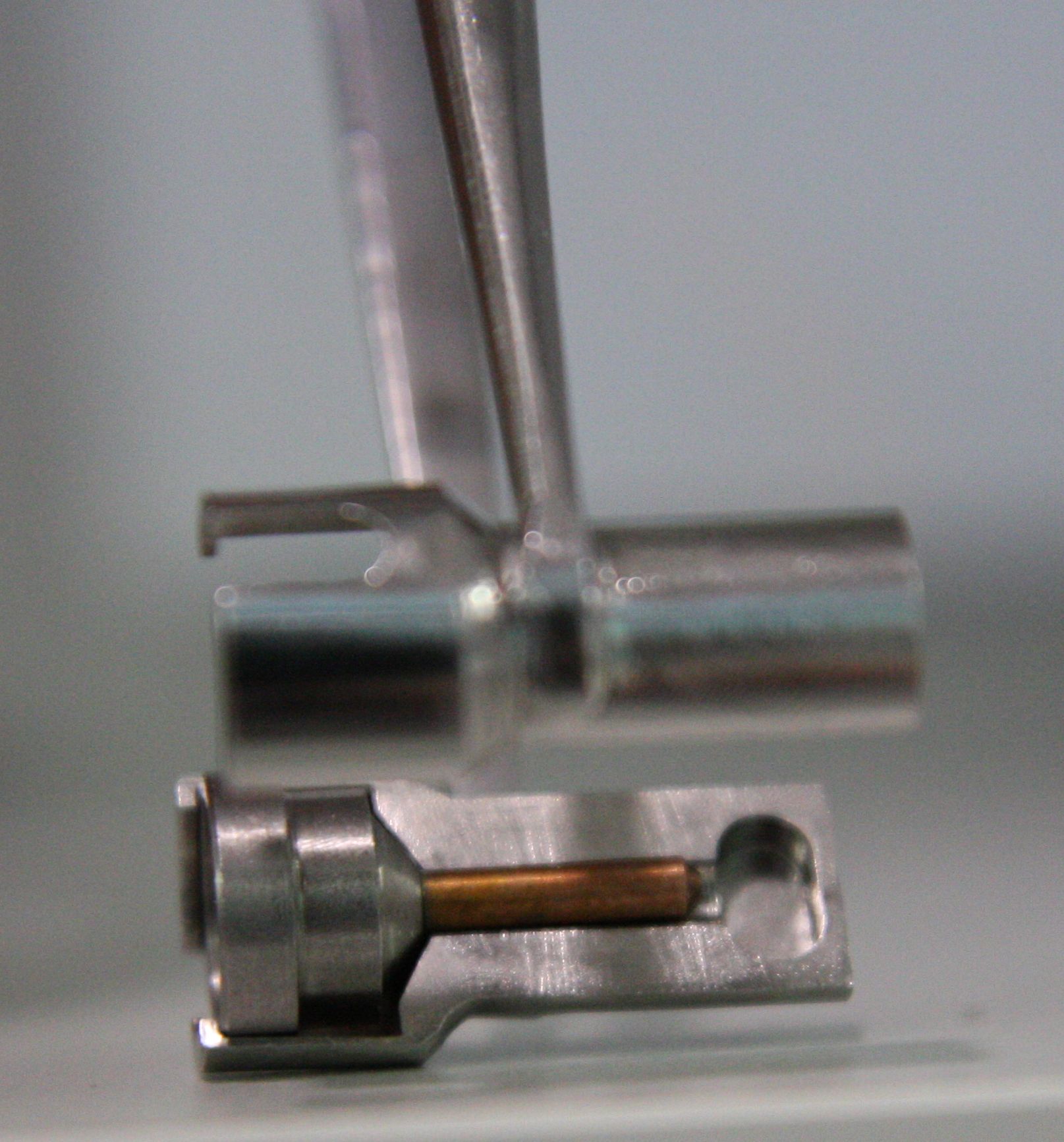 |
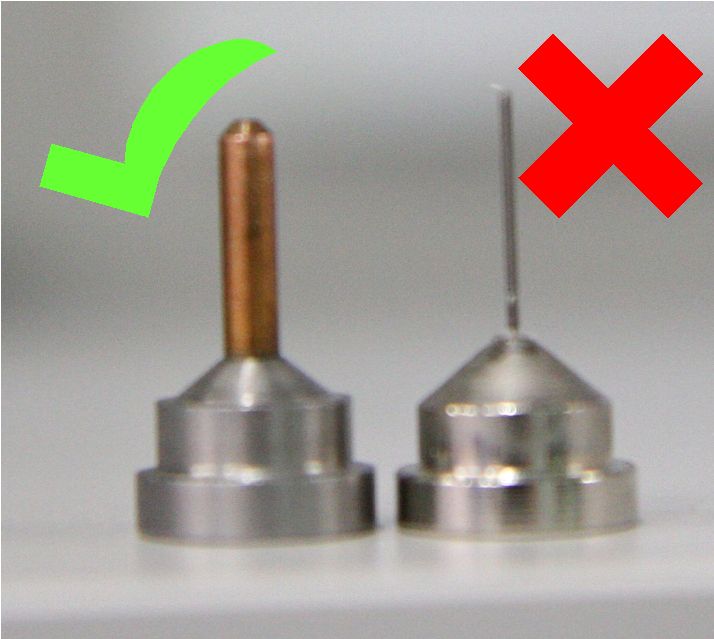 |
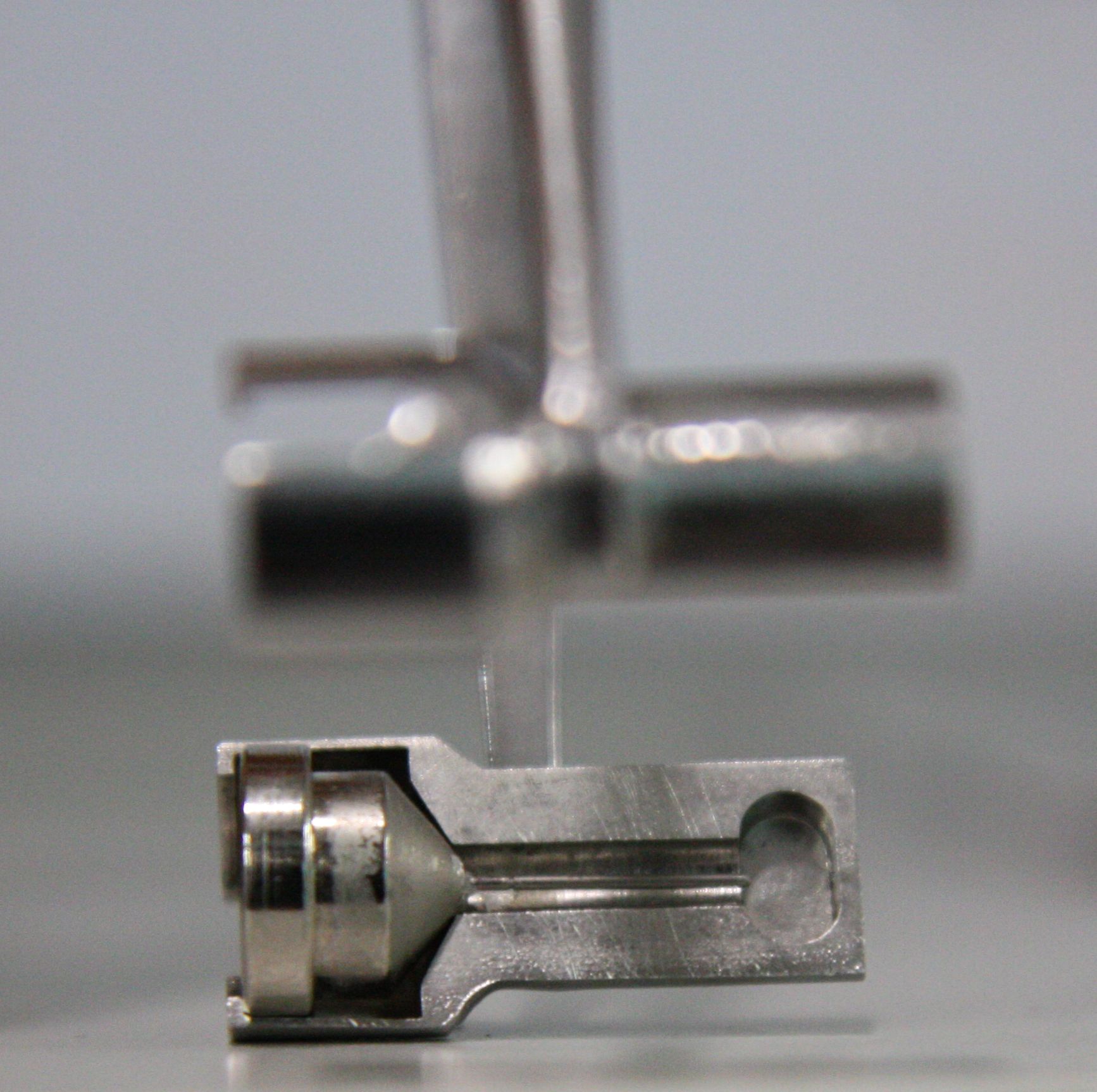 |
- Know the safety practices at the Australian Synchrotron for handling liquid nitrogen and follow the SOPs (Standard Operating Procedures) documents located on, or nearby, the equipment.
- Replace the liquid nitrogen in the shipping and loading dewars as soon as any ice forms.
Mounting options and ‘special techniques’ available on this beamline include:
- automated sample loading versus manual mounting directly onto the gonio. The automated loading technique involves using the robotic crystal loading machine; where samples are pre-loaded in a cylindrical cassette and placed on a mounting rod in the dewar such that users are able to mount and dismount samples using the Blu-Ice User Interface.
- In contrast, the manual option involves opening the hutch to manually mount/dismount samples directly on/off the gonio head. Note that both sample loading methods require use of the same kind of loop mount specified previously.
- Note also that the manual mounting option is unavailable when conducting remote access experiments.
- Optional magnetic sample mount for capillaries.
Detailed procedures for Manual Mounting and Auto (Robot) Mounting of crystals appear in the Beamline User Manual, which can be downloaded from the Resources webpage for this beamline.
On-Site Preparation Facilities
CRYOGENIC EQUIPMENT PROVIDED FOR USERS
The Macromolecular Crystallography beamlines (formerly known as the Protein Crystallography beamlines) have two liquid nitrogen LD35 Dewars (outside the beamline cabin) and one 4 litre ‘transfer’ dewar per beamline. In addition, each sample preparation area has durable foam rubber dewars that hold approximately 800ml of liquid nitrogen for loading crystals onto sample mounts. Tall versions of the foam rubber dewars can be used for transferring ‘canes’ between the users’ shipping dewar and smaller foam rubber vessels prior to mounting on the goniometer head.
|
|
|
The CrystalWand is a 205 mm chrome-plated steel wand (9.6 mm in diameter with a magnet molded inside one end) to assist with the transfer of crystals onto sample mounts under liquid nitrogen.
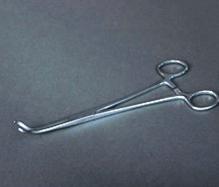
The Vial Clamp is a chrome plated, hemostat style tool. It has a tip shaped to hold the crystal storage vial at an angle of 45°/135° when the clamp is closed. The clamp can be locked using the hemostat style lock. The overall length of the clamp is 195 mm.
The CryoTong is used to manually transfer a crystal on a magnetic mount, from liquid nitrogen to the magnetic base on the goniometer head.
 |
 |
 |
 |
Full cryogenic PPE (face-shield, cryogenic gloves) are supplied for all users involved in dispensing liquid nitrogen. Safety glasses and nitrile gloves plus cotton (under-layer) gloves are supplied at each beamline for users handling liquid nitrogen when manually mounting crystals. Users are encouraged to bring their own cotton under-layer gloves if they have them.
LABORATORY EQUIPMENT PROVIDED FOR USERS
The following equipment is provided in both the PC1 Biochemical and Chemical Preparation Laboratories and can be used once users have read and understood the accompanying Standard Operating Procedures (SOPs):
- pH meter
- mini centrifuge
- sonication bath
- balances
- hotplate stirrers
- purified water system
- oven
- fridge and freezer
- microwave oven
- desiccator
- fumehoods
- optical microscopes (at each beamline).
LABORATORY GLASSWARE
There is a range of laboratory glassware and consumables available for use in both the biochemical and chemical preparation laboratories, including beakers, Erlenmeyer flasks, Petri dishes, pipettes and burettes. A small quantity of vials and plastic containers is available for ‘interim’ storage (during sample preparation), however users should endeavour to bring their own supply of high-volume or experiment-specific items.
Users should note that all vessels/flasks/vials/bottles etcetera must be clearly labelled with the experimenter’s name, date and the contents of the vessel. The contents of any unlabelled samples and vessels will be disposed of without prior warning.
CHEMICALS
Water, isopropanol, ethanol and acetone are provided in small quantities. If there are any specific chemical requirements for users’ experiments, the Principal Investigator is encouraged to contact the beamline support team to discuss arrangements for ordering/shipping with enough lead-time that the chemicals will be available and appropriately stored prior to the scheduled beamtime.
Hard copies of MSDSs for all chemicals stored in the Biochemistry Lab can be found in the accompanying folder on the chemical shelves. In addition, any chemical/s that users bring on-site with them must have MSDSs attached to their ESA and a copy located near their preparation station when using the laboratories for preparation of samples. Risk Assessments must be performed before starting any experimental work involving hazardous chemicals (as designated on the MSDS).
WATER PURIFYING SYSTEM
Nanopure RO and Nanopure Diamond (Milli-Q equivalent) water is available in the Biochemistry Lab. Card access to this laboratory must be arranged prior to users’ arrival by contacting the User Office and specifically requesting access to the labs.
SAMPLE FRIDGE/FREEZER
A fridge (4 °C) and freezer (-20 °C) are provided for users' samples. Ensure that all samples are adequately labelled with the users’ names, date and contents. Users MUST remove any stored items when they leave the Australian Synchrotron. Be aware that a ruthless disposal system will be in operation and, due to limited space, Synchrotron staff will not take responsibility for long-term storage of samples.
Transport & Safety
Click here for information on safe transport, handling and the need to use of a single selected company for reimbursement when shipping samples to AS beamlines.
SHIPPING DEWARS – INTERSTATE AND OVERSEAS USERS
It is highly recommended that interstate and overseas users transport their crystals to the Australian Synchrotron using a dry shipper so that the samples are guaranteed to arrive at the Australian Synchrotron at least one day in advance of beamtime (i.e. should leave the host institution no later than 4 days prior to beamtime). In a dry shipper the liquid nitrogen is captured in the special absorbent material surrounding the central core of the flask, producing a consistently dry but cold (-190°C) sample environment. A dry shipper does not rely on free liquid nitrogen for cooling and hence, even if the vessel is tipped over the liquid nitrogen does not spill and samples are not disrupted. In addition, the temperature of the dry shipper is easily maintained for long transport periods. However, it should be noted, that if shippers are delivered on site over the weekend, they may not be topped-up until the following Monday.
Upon arrival at the Australian Synchrotron delivery/loading bay, dry shippers will be topped-up with liquid nitrogen and moved to the beamline (weekdays only). The user support staff member on shift will then transfer crystal cassette/s to the robotic sample loader on the morning of the allocated beamtime and confirm the pin location/s in the robot dewar.
IMPORTANT: For more information on the documents needed to ship interstate and overseas, please consult our FAQ
"What do I need to do if I am shipping samples internationally?"
/index.php/aussyncbeamlines/macromolecular-crystallography/faqs-mx-beamlines
The Shipping Form contains the following:
- Exporter contact information – the user; This includes your name, address, phone number, company name and email. These details are required to import goods into Australia.
- Importer contact information – Australian Synchrotron; International users please note: Ensure that ALL samples shipped to the Australian Synchrotron are addressed to Dr Tom Caradoc-Davies. Otherwise your samples will not be permitted to be imported. i.e.
- Dr Tom Caradoc-Davies
Australian Synchrotron
MX Beamline
800 Blackburn Rd
Clayton
Melbourne
Australia 3168 - A detailed manifest of all your protein samples; Including, but not limited to: source, production organism and quantity.
International users who wish to bring samples to the Australian Synchrotron, or to ship them separately, are required to include with their samples a copy of the Australian Synchrotron's AQIS permit AND a letter from the Australian Synchrotron expressly approving their use of the permit for the date(s) of their experiment(s). International users will receive a signed, personalised copy of this letter, as well as a copy of the AQIS permit, from the User Office at the time that they are notified of their award of beamtime. If you have not received this then please contact the User Office.
The tracking number must be included in the Shipping Form. Also, make sure that you specify that you are doing a remote experiment by clicking on the corresponding check box and fill in the requested information for return of the shipper.
PLEASE NOTE: after your experiment the beamline staff will ensure that your samples are returned to your dry shipper and that the shipper is adequately emptied of liquid nitrogen for return. Staff will then take your dewar to our stores. THE AUSTRALIAN SYNCHROTRON STORES WILL ARRANGE UPLIFT OF THE DEWAR using the courier company and account details provided by the user on the online dewar shipping form. Please do not arrange your own courier uplift.
If you are not using a courier, you may take your own dewar with you at the end of your beam time. In doing so you take responsibility for ensuring that liquid nitrogen remaining in the dewar does not make it unsafe for transport and that your samples have been returned to the dewar from the beamlines. Please also scan the dewar out of the dewar database at the beamlines so that beamline staff know that the dewar has left the synchrotron. A staff member or operator can help you with this if you are unsure.
Waste Disposal
Click here for information about disposal of chemical waste.
CHEMICAL AND BIOLOGICAL WASTE
Users MUST have a safe disposal plan for any waste generated by their experiment/s before they start work.
Waste streams provided in the Biochemistry Lab include:
- water-soluble hydrocarbons
- water-insoluble hydrocarbons
- chlorinated hydrocarbons
- heavy metals
Please contact the user support team prior to your arrival if you require another waste stream.
SHARPS DISPOSAL
Sharps bins are provided on site at the beamline and in the Biochemistry Lab. These are for sharps ONLY: needles, glass, pipette tips, etcetera.
HOUSEKEEPING
The lab must be kept clean and tidy. Users are expected to clean up after themselves as there are no dedicated laboratory cleaners employed on-site. Lab privileges will be rescinded (deactivated on their access card) if a user group leaves the lab in an untidy or dirty state. Users should also note that cameras are installed in various locations around the facility, so poor housekeeping CAN be tracked.

.jpg)

