The Australian Synchrotron regularly seeks submissions for the Australian Synchrotron Thesis Medal. This medal is awarded annually to the PhD student at an Australian or New Zealand University who is judged to have completed the most outstanding thesis of the past two years - and whose work was undertaken at and acknowledges the Australian Synchrotron, or the Australian National Beamline Facility (ANBF), or whose work acknowledges and was undertaken under the auspices of the International Synchrotron Access Program (ISAP) or the ASRP.
The 2012 Australian Synchrotron Thesis Medal was awarded to Dr Miriam-Rose Ash, who completed her PhD studies at The University of Sydney and the Centenary Institute.
The medal was presented to Miriam-Rose's co-supervisor Mitchell Guss from The University of Sydney at the Australian Synchrotron User Meeting on 29 November 2012. Miriam-Rose is currently an EMBO postdoctoral research fellow at the Centre for Structural Biology at Aarhus University in Denmark.
 Read on for a summary of Miriam-Rose's work...
Read on for a summary of Miriam-Rose's work...
Potassium is a well-known and essential nutrient for human health. Potassium also plays a previously unsuspected but essential role in the bacteria behind illnesses such as gastroenteritis and Legionnaires’ disease. Miriam-Rose Ash, the 2012 synchrotron thesis medal winner, explains:
In order to survive within the human body, bacteria must scavenge iron – a metal essential for almost all life on earth – from their immediate environments. One way that this is achieved is through the protein FeoB, which resides in the inner membranes of bacteria and facilitates the uptake of ferrous ions (Fe2+). Because of its role in iron acquisition, this protein is essential for infection by bacteria that cause illnesses such as gastroenteritis and Legionnaires’ disease in humans.
Photo: Miriam-Rose Ash with the Governor of NSW, Prof. Marie Bashir
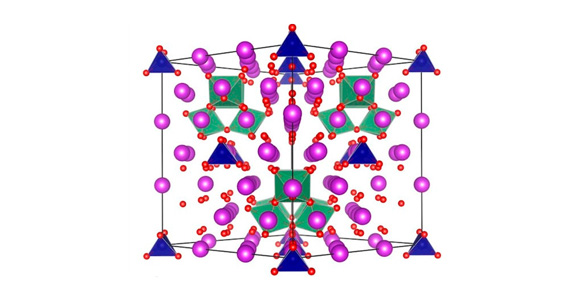
Transport of ferrous ions by FeoB requires the activity of its G-protein domain, which binds to and hydrolyses the nucleotide GTP (guanosine triphosphate). When the rate of this reaction was measured on purified protein outside of the cell, it was found to be too slow to permit iron uptake at the speed required for bacterial survival. The apparently slow speed of this reaction therefore remained a puzzle for many years, and researchers strove to understand how the rate of GTP hydrolysis might be accelerated inside the cell. The work conducted at the Australian Synchrotron finally shed some light on this mystery. Using synchrotron radiation, the 3D structure of the G-protein domain of FeoB was determined in complex with a transition-state analogue of GTP. This structure revealed a very distinctive conformation of the protein around the nucleotide and an unexpected addition at the nucleotide binding site: a potassium ion.
Potassium is one of the must abundant cations inside the cell, and its presence at the nucleotide binding site in FeoB indicted that it might play a role in the protein’s function. When the rate of GTP hydrolysis was measured in the presence of potassium, which was not included in any earlier experiments on the protein, nucleotide hydrolysis occurred much faster than ever before. This work helped to explain the mechanism of GTP hydrolysis by FeoB, and also suggested that the reaction could indeed proceed fast enough inside the cell to facilitate iron uptake by the protein and permit bacterial infection.
However, the structure of the G-domain of FeoB had even more surprises in store. By comparing the structure and sequence of FeoB with those of other G-proteins, some revealing similarities soon emerged. It was found that the unique structure of the protein around the nucleotide binding site was also observed in other G-proteins, and furthermore, the particular amino acid residues involved in potassium ion binding were also conserved. Together, these two things suggested that a host of other G-proteins – involved in essential biological process in all organisms – will also exhibit potassium-dependent GTP hydrolysis activity. In this way, the structure of the G-protein domain of FeoB not only gave insight into the mechanism of iron uptake in bacteria, but also effectively redefined the conditions under which many other G-proteins should be studied in the future.