 Producing molecular hydrogen from water could provide a sustainable energy supply for the future, but commercial applications are hampered by the cost of expensive platinum catalysts.
Producing molecular hydrogen from water could provide a sustainable energy supply for the future, but commercial applications are hampered by the cost of expensive platinum catalysts.
A metal-free catalyst developed by an Australian-international research team may hold the answer.
Currently, the most effective way to produce hydrogen from water involves using platinum catalysts that provide a surface to temporarily ‘hold’ hydrogen ions (protons) liberated from water molecules so that two protons can combine with two electrons to form a hydrogen gas H2 molecule.
An international research team involving three Australian universities has used the Australian Synchrotron to investigate the properties of a metal-free electrocatalyst that can produce similar amounts of hydrogen to some of the well-developed metallic alternatives to platinum catalysts. The catalyst combines graphitic-carbon nitride (g-C3N4) with nitrogen-doped graphene (NG), both of which form regular 2D structures that can be stacked or joined to make larger arrays.
The collaborators believe this is the world’s first metal-free hybrid catalyst for electrocatalytic evolution of hydrogen. Its exciting electrocatalytic properties appear to stem from chemical bonds and other interactions between the graphitic-carbon nitride and the nitrogen-doped graphene components.
The Australian researchers involved in this work are from the universities of Adelaide and Queensland, Deakin University and the Queensland University of Technology. Their collaborators are from King Abdullah University of Science and Technology in Saudi Arabia and Kent State University, Ohio, USA.
The researchers used the Synchrotron’s soft x-ray absorption spectroscopy beamline to probe the interactions between the graphitic-carbon nitride and the nitrogen-doped graphene. They used two synchrotron techniques: near-edge x-ray absorption fine structure (NEXAFS) and x-ray photoelectron spectroscopy (XPS).
In their recent paper in Nature Communications, the researchers say their findings provide clear evidence that “well-designed metal-free counterparts ... have great potential for highly efficient electrocatalytic evolution of hydrogen”, similar to precious metals. The interactions that take place between graphitic-carbon nitride and nitrogen-doped graphene open up “a new avenue towards replacing noble metals by broader alternatives in a wide variety of applications”.
Nature Communications, 5, 3783 (2014)
http://www.nature.com/ncomms/2014/140428/ncomms4783/full/ncomms4783.html
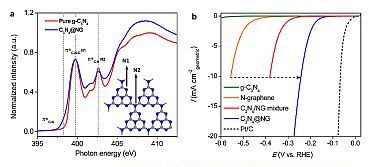
a) NEXAFS spectra for pure graphitic carbon-nitride (g-C3N4) and the hybrid electrocatalyst C3N4@NG show different environments for nitrogen atoms in the structure.
b) Hydrogen evolution reaction (HER) polarisation curves show that the hybrid electrocatalyst C3N4@NG performs better than the component materials, but not as well as a 20% platinum/carbon electrocatalyst.
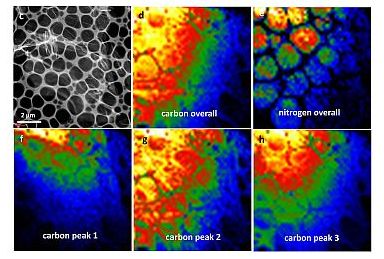
c-h) Scanning transmission electron microscopy images of C3N4@NG nanosheet. In these colour-coded images, intensity increases as the colour changes from black to blue to green to red to yellow to white.
